|
|
||||||||||||||||
|
||||||||||||||||
|
||||||
|
|
|
|
Boracite
|
|
| | |
| Discovered in 1789; IMA status: Valid (pre-IMA; Grandfathered) | ||
|
| ||
|
Chemistry |
|
|
| |
|
Mg3B7O13Cl | |
|
|
Magnesium Borate Chloride |
|
Molecular Weight: |
392.03 gm |
|
Composition: |
Magnesium |
18.60 % |
Mg |
30.84 % |
MgO |
|
|
Boron |
19.30 % |
B |
62.15 % |
B2O3 |
|
|
Chlorine |
9.04 % |
Cl |
9.04 % |
Cl |
|
|
- |
- % |
Cl |
-2.04 % |
-O=Cl2 |
|
|
Oxygen |
53.06 % |
O |
|
|
|
|
|
100.00 % |
|
100.00 % |
= TOTAL OXIDE |
|
|
|
||||
|
Classification |
|
|
| |
|
Borates | |
|
5/L.04-10 | |
|
|
6 : BORATES
|
|
Related to: |
Boracite Group. Boracite-Ericaite Series. The magnesium analogue of Ericaite and Chambersite. The orthorhombic dimorph of Trembathite. |
|
Members of Group: |
Boracite Group: Boracite, Chambersite, Congolite, Ericasite, Trembathite. |
|
Varieties: |
Bromboracit, Ferroan Boracite, Stassfurtite |
|
Synonyms: |
Alpha-boracite, b-Boracite, Beta-boracite, Boracite (of Werner), Borate of Magnesia, Metaboracite, Parasite |
|
|
|
|
Crystal Data |
|
|
|
|
|
Euhedral crystals, to 2.5 cm, (referred to pseudotetrahedral morphology) and a dozen other modifying forms; spherulitic, plumose to fibrous, fine granular aggregates. |
|
|
Rarely as penetration twins |
|
|
|
|
|
Physical Properties |
|
|
|
|
|
None observed |
|
|
Conchoidal to Irregular/Uneven |
|
|
Brittle |
|
|
7.0 - 7.5 |
|
|
2.91 - 3.10 (g/cm3) |
|
|
None |
|
|
Not Radioactive |
|
|
Other: |
Strongly piezoelectric and pyroelectric. Very slowly decomposed by water. Slowly but completely soluble in HCl. At 265° the crystal system reverts to a high-temperature phase and the material becomes isotropic. Forms pseudomorphs after quartz (Douglashall). |
|
Optical Properties |
|
|
|
|
|
Pale green, greenish blue, blue, colorless, grey, white; dark green (ferroan) |
|
|
Transparent, Translucent |
|
|
Adamantine to Vitreous |
|
|
1.658 - 1.673 Biaxial ( + ) |
|
|
0.010 - 0.011 |
|
|
0.024 (weak) |
|
|
None |
|
|
|
|
|
Occurances |
|
|
|
|
|
Geological Setting: |
Bedded sedimentary deposits of gypsum and anhydrite; salt deposits; potash deposits of the oceanic type. |
|
Common Associations: |
Anhydrite, Carnallite, Danburite, Gypsum, Halite, Hilgardite, Kainite, Magnesite |
|
Common Impurities: |
Fe(II) |
|
Type Locality: |
Kalkberg hill, Lüneburg, Lower Saxony, Germany |
|
Year Discovered: |
1789 |
|
View mineral photos: | |
|
|
|
|
Unusual Gem Categories |
|
|
|
|
|
| |
|
|
|
|
More Information |
|
|
|
|
|
| |
|
|
|
|
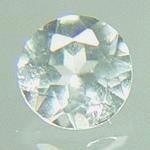
Boracite is one of the rarest of collector's gems. The only facetable crystals come from the Stassfurt and Hanover districts of Germany. Boracite crystals are very small and cut gems are usually very pale blue to green or colorless. Boracite is the magnesium analogue of Chambersite. Boracite is an evaporite mineral and is not surprisingly found with other evaporite minerals like Anhydrite, Gypsum and Halite. Boracite crystals are often embedded in these other evaporite minerals. Boracite is one of the rare minerals that exhibit both the piezoelectric effect and the pyroelectric effect. Piezoelectricity is the ability of some mineral crystals to generate a voltage in response to applied mechanical stress such as an external pressure or stress. Piezoelectricity was discovered in 1880 by French physicists, brothers Jacques and Pierre Curie. Pyroelectricity is the ability of certain mineral crystals to generate an electrical charge when they are heated or cooled. The first reference to the pyroelectric effect is in writings by Theophrastus in 314 BC, who noted that Tourmaline becomes charged when heated. Sir David Brewster gave the effect the name it has today in 1824. Both William Thomson in 1878 and Voight in 1897 helped develop a theory for the processes behind pyroelectricity. Boracite is also a water soluble mineral. Boracite distribution: in Germany, from Lüneburg, 40 km south-southeast of Hamburg, Lower Saxony; in Saxony-Anhalt, in the Stassfurt-Westeregeln-Bernburg district, at the Douglashall, Berlepsch, Solvayhall, Wilhelmshall and other mines; in Thuringia, from Bischofferode, in the Glückauf mine, Sondershausen, and elsewhere. In France, at Lunéville, Meurthe-et-Moselle. In the Boulby potash mine, northwest of Whitby, Yorkshire, England. In the Chelkar salt dome, Ak-sai Valley, Uralsk district, Kazakhstan. Large crystals from Alto Chapare, Cochabamba, Bolivia. In the USA, in the Choctaw salt dome, Iberville Parish, Louisiana, and the Louann Salt Formation, Clarke County, Alabama. From the Penobsquis and Salt Springs evaporite deposits, near Sussex, New Brunswick, Canada. |
|
|
Boracite gems for sale:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||